Executive Committee
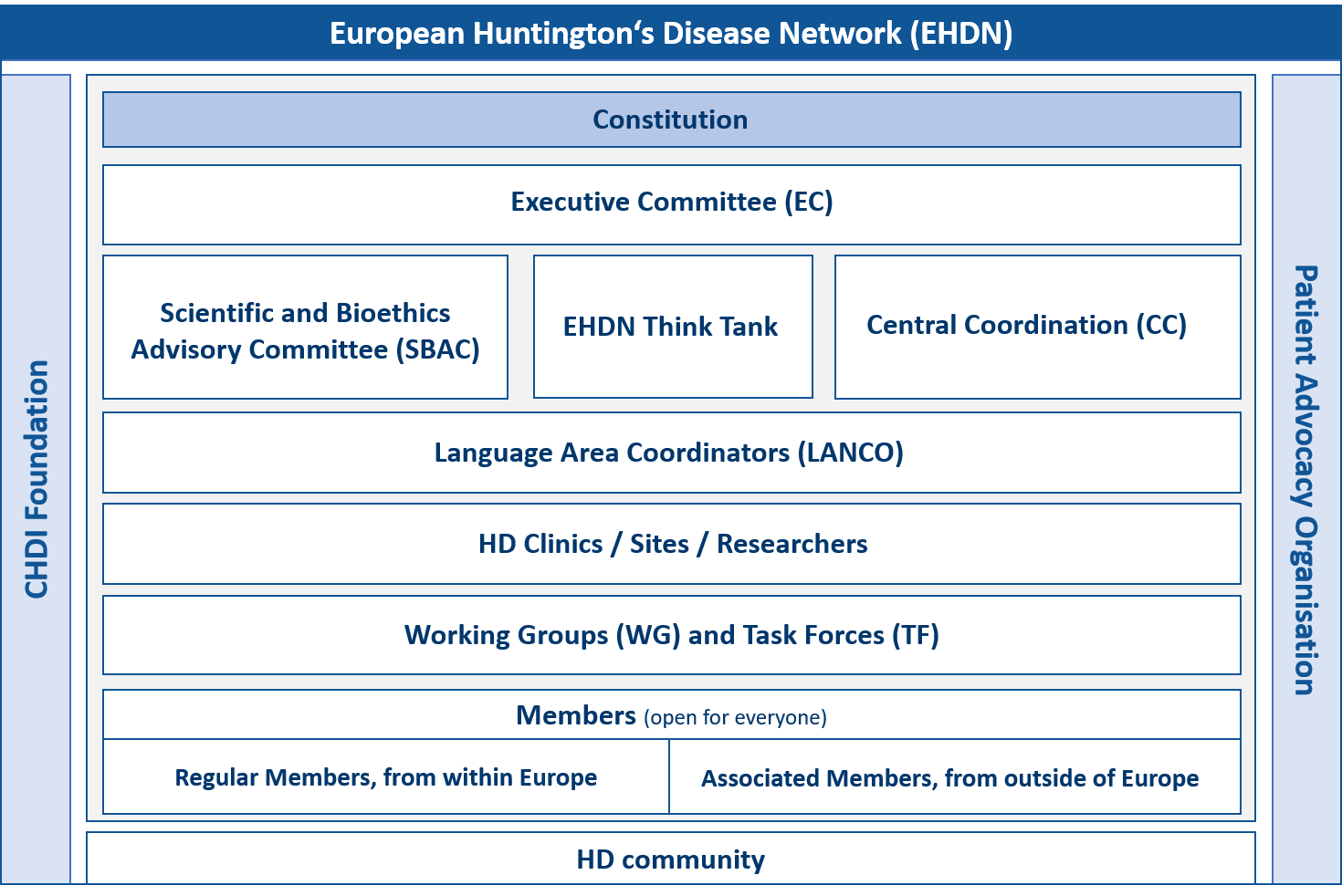
The Executive Committee (EC) is the body responsible for governing the EHDN, directing and overseeing its activities and establishing its strategy. The EC meets monthly and endorses clinical trials, approves access to clinical data and biosamples from the Registry study, and approves seed fund applications twice per year. Its members are elected by majority vote of the EHDN membership for a term of four years. For more information on the EC, please refer to article 4 of the constitution.
Members of the Executive Committee
Central Coordination
Central Coordination (CC) is the central hub of the EHDN. As the core of all operational activities it ensures the efficient functioning of the EHDN at the behest of the EC. Its physical headquarters are located at the University Hospital of Ulm in Germany, although most CC staff are dispersed throughout Europe. The EHDN operates as a virtual network, meaning that its staff meet regularly via teleconference or Skype and conduct face-to-face meetings several times per year. CC oversees clinical operations, science management, legal and financial administration, project management, IT coordination, training coordination and grant management.
Members of Central Coordination
Language Area Coordinators
Language Area Coordinators (Lancos) are the bridge between the EHDN and its clinical sites throughout Europe. If CC is the heart of the network, Lancos are the arteries that funnel support, advice and assistance to the 150+ sites that make up the EHDN’s clinical network. Lancos are located in their respective language areas, where they provide support to study sites and liaise with local patient advocacy grops. Lancos monitor Enroll-HD and Registry data and visit sites on a regular basis to build relationships with site staff and better understand specific activities and areas of concern. In other words, they mediate between the EHDN and the study sites in a mutually beneficial way. Other Lanco activities include translating the written materials generated by the EHDN into their respective languages, organising annual site investigator meetings in their regions, assisting sites with ethical review board or ethical committee submissions, and assisting sites in the execution of EHDN-endorsed clinical trials.
Members of Language Coordination
Governance
The constitution of the EHDN governs its mission, principles and activities, as well as the rights and responsibilities of its elected committees, staff and members. The original constitution was drafted by the Constitution Working Group in 2005-6 and adopted by majority vote of the EHDN membership at the plenary meeting in Blankenberge, Belgium in September 2006. The constitution was subsequently amended by majority vote at the plenary meeting in Stockholm in September 2012 and then during the virtual meeting in September 2021.
Constitution
Scientific and Bioethics Advisory Committee
The Scientific and Bioethics Advisory Committee (SBAC) meets on a monthly basis and is responsible for reviewing research projects submitted to the EHDN that require access to data and biosamples from Registry, or funding under the seed fund scheme. The SBAC sends its recommendations to the EC for further consideration and approval. The SBAC also reviews trial protocols submitted to the EHDN for endorsement. Its members are elected by majority vote of the EHDN membership for a term of four years. For more information on the SBAC, please refer to article 5 of the constitution.









































































